Physiology
Research outline
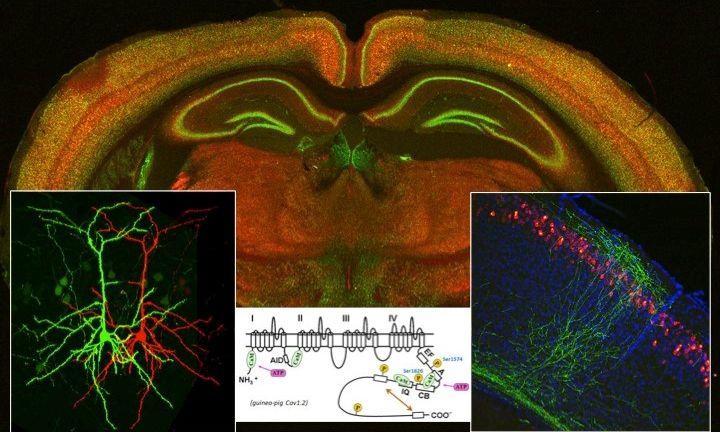
Our brain and heart function using changes in membrane potential (electrical signals). How is the electrical signal generated and regulated? How do neurons in the brain use such electrical signals for their circuit formation during development? What happens if the regulation of membrane potentials goes wrong? We address these questions using state-of-the-art techniques in molecular biology, electrophysiology, and imaging.
| Graduate School | Graduate School of Medical and Dental Sciences |
|---|---|
| Course | Advanced Therapeutics Course |
| Field | Neurology |
| Department | Physiology |
| Homepage | http://www.kufm.kagoshima-u.ac.jp/~physiol2/ |
Contact
Research interests
-
Activity-dependent formation of neural circuits in the cerebral cortex
-
Restoration of damaged circuits
-
Function and dysfunction of ion channels
Staff

Professor
| Name | Yoshiaki TAGAWA |
|---|---|
| Specialized field | Neuroscience |
| Research interests | Neural circuits in the cerebral cortex |

Lecturer
| Name | XU Jianjun |
|---|---|
| Specialized field | Physiology of Ion channels, Molecular Biology |
| Research interests |
|

Lecturer
| Name | Etsuko MINOBE |
|---|---|
| Specialized field | Physiology of Ion channels, Molecular Biology |
| Research interests |
|

Assistant Professor
| Name | Nao Nakagawa-Tamagawa |
|---|---|
| Specialized field | Neuroscience |
| Research interests |
|
Research results
Yoshiaki TAGAWA
Developmental stage-specific spontaneous activity contributes to callosal axon projections . eLife11 ( e72435 ) 1 - 15 2022
Long-Range Interhemispheric Projection Neurons Show Biased Response Properties and Fine-Scale Local Subnetworks in Mouse Visual Cortex . Cerebral Cortex31 ( 2 ) 1307 - 1315 2021
Involvement of Calcium-Dependent Pathway and β Subunit-Interaction in Neuronal Migration and Callosal Projection Deficits Caused by the Cav1.2 I1166T Mutation in Developing Mouse Neocortex. Frontiers in Neuroscience, 15, 747951, 2021
Long-range interhemispheric projection neurons show biased response properties and fine-scale local subnetworks in mouse visual cortex. Cerebral Cortex, 31(2), 1307–1315, 2020.
自発神経活動と視覚系神経回路のスクラップ&ビルド 生体の科学, 70, 23-27, 2019.
Control of spontaneous Ca2+ transients is critical for neuronal maturation in the developing neocortex. Cerebral Cortex, 26, 106-117 2016.
Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nature Neuroscience, 18(12), 1780-1788 2015.
Dysfunction of KCNK potassium channels impairs neuronal migration in the developing mouse cerebral cortex. Cerebral Cortex, 24, 1017-1029 2014.
Evidence for activity-dependent cortical wiring: Formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. Journal of Neuroscience 27(25), 6760-6770, 2007.
Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience 8(3), 380-388, 2005.
XU Jianjun
Regulation of cardiac Cav1.2 channels by calmodulin. International Journal of Molecular Sciences, 24 (7) ( 6409 ), 1-28, 2023.
Ca2+ Dyshomeostasis Links Risk Factors to Neurodegeneration in Parkinson’s Disease. Front Cell Neurosci., 16:867385, 2022.
Purification of insoluble GST-fused and GST-cleaved Cav1.2 channel fragment by denaturation and renaturation. Protein Expr Purif.,160: 7-10, 2019.
PKA phosphorylation of Cav1.2 channel modulates the interaction of calmodulin with the C terminal tail of the channel. J Pharmacol Sci.,137(2):187-194, 2018.
A new interaction between proximal and distal C-terminus of Cav1.2 channels. J Pharmacol Sci.,133(4):240-246, 2017.
PKA and phosphatases attached to the Cav1.2 channel regulate channel activity in cell-free patches. American Journal of Physiology Cell Physiology, 310(2), C136-141, 2015.
Calmodulin reverses rundown of L-type Ca2+ channels in guinea pig ventricular myocytes. American Journal of Physiology Cell Physiology, 287(6), C1717-24, 2004.
Etsuko MINOBE
Regulation of cardiac Cav1.2 channels by calmodulin. International Journal of Molecular Sciences, 24 (7) ( 6409 ), 1-28, 2023.
Ca2+ Dyshomeostasis Links Risk Factors to Neurodegeneration in Parkinson’s Disease . Frontiers in Cellular Neuroscience16 ( 867385 ) 1 - 15 2022
Properties of Calmodulin Binding to Nav1.2 IQ Motif and Its Autism-Associated Mutation R1902C . Neurochemical Research46 ( 3 ) 523 – 534, 2021
Calmodulin and ATP support activity of the Cav1.2 channel through dynamic interactions with the channel. The Journal of Physiolgy, 595(8), 2465-2477, 2017.
A new phosphorylation site in cardiac L-type Ca2+ channels (Cav1.2) responsible for its cAMP-mediated modulation. American Journal of Physiology Cell Physiology, 307, C999-C1009, 2014.
Calpastatin domain L is a partial agonist of the calmodulin-binding site for channel activation in Cav1.2 Ca2+ channels. The Journal of Biological Chemistry, 286, 39013-39022, 2011.
A region of calpastatin domain L that reprimes cardiac L-type Ca2+ channels. Biochemical and Biophysical Research Communications, 348, 288-294, 2006.
Nao Nakagawa-Tamagawa
Complex activity and short-term plasticity of human cerebral organoids reciprocally connected with axons. Nature Communications 15, 2945, 2024
In Vivo Visualization of Spontaneous Activity in Neonatal Mouse Sensory Cortex at a Single-neuron Resolution . Journal of Visualized Experiments ( 201 ) 2023
In vivo two-photon calcium imaging of cortical neurons in neonatal mice . STAR protocols 4(102245) 2023
Involvement of Calcium-Dependent Pathway and β Subunit-Interaction in Neuronal Migration and Callosal Projection Deficits Caused by the Cav1.2 I1166T Mutation in Developing Mouse Neocortex. Frontiers in Neuroscience, 15, 747951, 2021
Slow Dynamics in Microcolumnar Gap Junction Network of Developing Neocortical Pyramidal Neurons. Neuroscience 406(2019) 554-562, 2019
Lattice system of functionally distinct cell types in the neocortex.Science 358(6363), 610-615. 2017
Schizophrenia-like phenotypes in mice with NMDA receptor ablation in intralaminar thalamic nucleus cells and gene therapy-based reversal in adults.Translational psychiatry 7(2) e1047. 2017
Identity of neocortical layer 4 neurons is specified through correct positioning into the cortex.eLife 5. pii: e10907. 2016
Collaborations
Kyoto University, Kyusyu University, The University of Tokyo, National Institute of Genetics, Sumitomo Dainippon Phama
Research Grant List
Yoshiaki TAGAWA
| Project・Event / Span | Research |
|---|---|
| 科研費 基盤研究(C) 代表 2021-2023年度 |
大脳の投射先特異的な神経回路構築機構の解明 |
| 科研費 新学術領域研究公募 代表 2019-2020年度 |
神経活動に依存したスクラップアンドビルドによる大脳長距離神経回路の形成 |
| 科研費 新学術領域研究公募 代表 2017~2018年度 | 自発神経活動に依存した大脳長距離軸索投射の形成・再編・再生 |
| 科研費 基盤研究(C) 代表 2016~2018年度 | 同期・非同期の自発神経活動による大脳皮質神経回路の形成とその障害 |
| 科研費 新学術領域研究公募 代表 2013~2014年度 | 哺乳期マウスの神経活動操作・記録実験による生後初期神経活動の役割の解明 |
| 戦略的創造研究推進事業CREST 分担 2010~2016 | 大脳皮質の機能的神経回路の構築原理の解明 |
| 科研費 基盤研究(C) 代表 2011~2013年度 | 活動依存的メカニズムに基づく大脳皮質長距離軸索投射の再建 |
| 科研費 新学術領域研究公募 代表 2011~2012年度 | 皮質2/3層興奮性細胞の特徴的な形態・機能獲得における神経活動の役割 |
| 科研費 若手研究(B) 代表 2009~2010年度 | 大脳皮質の層特異的回路網構築における神経活動の役割 |
| 科研費 若手研究(B) 代表 2006~2008年度 | Cross-modal可塑性における代謝型グルタミン酸受容体の役割の解明 |
XU Jianjun
| Project・Event / Span | Research |
|---|---|
| 科研費 基盤研究(C) 代表 2019-2021年度 |
Molecular mechanism of self-regulation of Cav1.2 channel by channel cytoplasmic fragments |
| 科研費 基盤研究(C) 一般、分担 2015-2017年度 |
L型Caチャネル調節機構の総合的研究 |
| 科研費 基盤研究(C)一般、分担 2007~2008 |
L型CaチャネルのCa依存性促進と不活性化の分子機構 |
Etsuko MINOBE
| Project・Event / Span | Research |
|---|---|
| 科研費 基盤研究(C)分担 2007~2008 |
L型CaチャネルのCa依存性促進と不活性化の分子機構 |
| 科研費 若手研究(B)代表 2009~2010 |
L型Caチャネルの活性制御におけるカルモジュリンの役割とその調節機構 |
| 科研費 基盤研究(B)分担 2009~2011 |
心筋Caチャネルの調節機構間相互作用 |
| 科研費 若手研究(B)代表 2011~2012 |
カルモジュリンをリンクさせた変異体を用いたL型Caチャネルの活性調節機構 |
| 科研費 基盤研究(C)代表 2013~2015 |
カルモジュリンによるL型Caチャネルの活性制御の分子機構の解明 |
| 科研費 基盤研究(C)代表 2016~2018 |
Cav1.2チャネルの活性とカルモジュリン結合部位との機能的相関の解明 |
| 科研費 基盤研究(C) 代表 2019-2021年度 |
2分子のカルモジュリンによるCav1.2チャネル不活性化機構の解明 |
| 科研費 基盤研究(C) 代表 2022-2024年度 |
カルモジュリンに制御されるCav1.2チャネルの2つの不活性化機構 |
Nao Nakagawa-Tamagawa
| Project・Event / Span | Research |
|---|---|
| 科研費 基盤研究(B) 分担 2024-2027年度 |
生体光遺伝子を用いた同期的神経活動の誘発による脳回路発達の改善 |
| 科研費 学術変革領域研究(A) 公募研究 代表 2024-2025年度 |
細胞内分子構造動態解析による神経細胞極性の決定・維持メカニズム解明 |
| 科研費 基礎研究(C) 分担 2023-2025年度 |
大脳皮質視覚野臨界期の神経可塑性における乳酸代謝の寄与の解明 |
| 科研費 基盤研究(C) 代表 2022-2024年度 |
発達期の神経活動レベル調節による大脳半球間投射制御の解明 |
| 科研費 学術変革領域研究(B) 分担 2022-2024年度 |
発達期大脳における多元自発活動と回路形成の因果関係の解明 |
| 科研費 基盤研究(C) 分担 2020-2022年度 |
新生仔生体イメージングによる大脳皮質回路形成における自発的同期活動の役割の解明 |
| 科研費 基盤研究(C) 代表 2019-2021年度 |
細胞種特異的な神経回路形成への発達期gap junctionネットワークの役割 |
| 科研費 挑戦的萌芽研究 代表 2016~2018 |
大脳新皮質第5層のgap junctionネットワークによる皮質内基本構造形成 |